Abstract
Background/Hypothesis: Children and adults with sickle cell anemia (SCA) exhibit decreased cardiopulmonary fitness. Anemia is directly related to oxygen carrying capacity and is one factor that affects cardiopulmonary fitness. The new sickle cell drug voxelotor raises hemoglobin in patients with SCA treated or untreated with hydroxyurea. We hypothesized that voxelotor improves exercise capacity in youths with SCA.
Methods: A single-center, open-label, single-arm longitudinal interventional pilot study was conducted for patients with SCA age > 12. Participants performed baseline Cardiopulmonary Exercise Testing (CPET#1), took 1500mg voxelotor for 2 months, then CPET was repeated (CPET#2). A modified Bruce Protocol using 2-minute stages was performed on a motorized treadmill, for a goal of 8-12 minutes of exercise. Breath by breath gas exchange data were collected and analyzed using a VMax Encore 29C metabolic cart. The metabolic test included standard monitoring of heart rate, EKG ST changes, arrhythmias, and O2 saturation. A respiratory quotient >1.1 was used as evidence of participant effort. Peak oxygen consumption (peak VO2), anaerobic threshold (AT), O2 pulse, VE/VCO2 slope, and time exercised in CPET#1 and CPET#2 were compared for each participant. The primary endpoint was peak VO2.
Hemoglobin (Hgb), reticulocyte count, and bilirubin were measured before CPET#1 and CPET#2. Pill count was used to monitor medication adherence and left shift of the P50 oxygen dissociation curve was used to document biochemical effect of voxelotor. Patient Global Impression of Change (PGIC) and Clinician Global Impression of Change (CGIC) surveys were collected at the end of the study.
Statistical analysis was performed using Student's paired T-test.
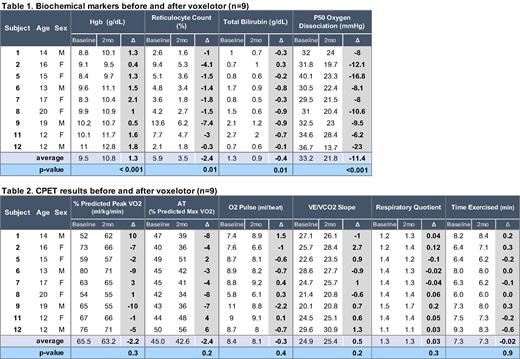
Results: Nine SCA patients ages 12-20, including 4 males and 5 females, completed the study. All had Hgb SS and were stably maintained on hydroxyurea, which was continued without dose change during the study. After 2 months of voxelotor, all participants demonstrated expected hematologic changes, including mean rise in Hgb +1.3 g/dL (95% C.I.= 0.8, 1.7), mean decrease in reticulocyte count -2.4% (95% C.I.= -4.1, -0.8), and mean decrease in bilirubin -0.4 mg/dL (95% C.I.= -0.8, -0.1). All participants demonstrated voxelotor adherence and leftward shift of p50 (Table 1).
Oxygen consumption, measured as percent predicted peak VO2 (ml/kg/min), ranged from 52% to 80% in CPET#1 and from 55% to 71% in CPET#2. The changes in peak VO2 for individual participants ranged from -10% to +10% of predicted peak VO2, with a mean difference of -2.2% (95% CI = -7.1, 2.7), which is insignificant (p=0.3). Using +/- 6% as variability in peak VO2 measurement, 5 participants exhibited no change, 3 participants had decrease in peak VO2 of -7%, -9%, and -10%, while peak VO2 increased by +10% for a single participant, who started to exercise on his own after starting voxelotor. Changes in individuals' anaerobic threshold, O2 pulse, VE/VCO2 slope, and time exercised were not significant and did not correlate with changes in peak VO2. All participants achieved respiratory quotient >1.1, assuring participant effort during CPET (Table 2).
On the 7-point PGIC questionnaire evaluating activity limitations, symptoms, emotions, and overall quality of life, 7 out of 9 participants reported positive change, including "a great deal better," "definite," and "moderate" improvements. Two participants reported minimal to no change, and no participants reported worsening. In comparison, clinicians reported "minimal" to "much" improvement on CGIC for all participants. Overall, patient impression of improvement was higher than clinician impression of improvement.
Conclusion: This pilot study demonstrated the feasibility of using CPET to evaluate exercise capacity longitudinally in youths with SCA. After addition of voxelotor to hydroxyurea for 2 months, all patients perceived global improvement. Peak VO2 did not change in 8 out of 9 participants and improved for 1 participant who exercised between the 2 CPETs. To increase peak VO2, higher Hgb increase, concurrent regular exercise, and longer exposure to voxelotor may be necessary.
This study was funded by Global Blood Therapeutics
Larkin: Forma Therapeutics, Inc.: Research Funding. Dulman: Pfizer: Other: own stock. Kuypers: Forma Therapeutics, Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal